Pediatric Precision Medicine and Experimental Therapeutics
This group belongs to the UMR 1015 "Tumour Immunology and anti-cancer immunotherapy".
The aim of our group is to develop new innovative therapies for children with advanced cancers through our clinical precision cancer medicine trials, with one group focusing on osteosarcoma classification and development of new innovative adapted therapies in children, adolescent and young adults.
Research topics
The mission of our research team is to identify innovative therapeutic strategies and targets for advanced pediatric tumors.
The first axis on pediatric cancer precision medicine led by B Geoerger and J Salmon builds on the MOSCATO-01-Ped (Harttrampf et al. 2017) and MAPPYACTS clinical trials (NCT02613962), in which a comprehensive characterization of the genetic alterations, transcriptional profiles, and immunological properties of refractory and recurrent pediatric tumors have been established through large-scale exome and RNA sequencing of tumor biopsies and bioinformatic analyses.
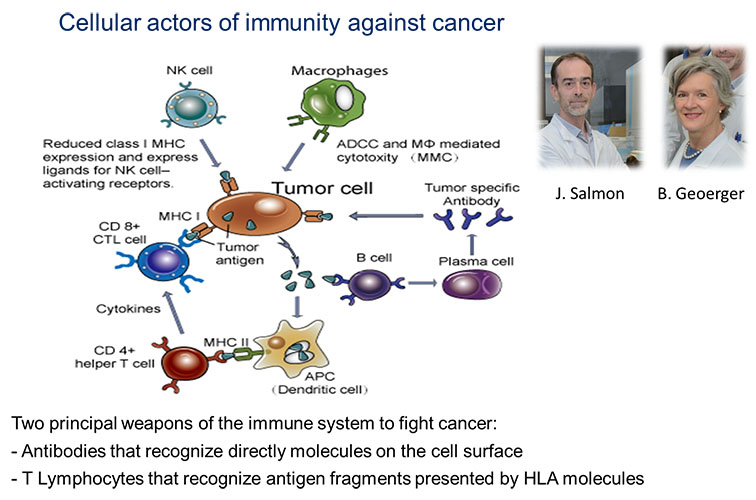
The major goal is to identify druggable targets, particularly antigens expressed de novo, overexpressed, or specifically mutated in tumors, which could be present at the tumor cell surface either as bona fide membrane proteins or as human leukocyte antigen (HLA)/peptide complexes and could be targeted by antibody- or T cell-based therapeutic approaches, respectively, as well as candidate immuno-oncology targets that could be expressed in the tumor microenvironment and contribute to immune escape.
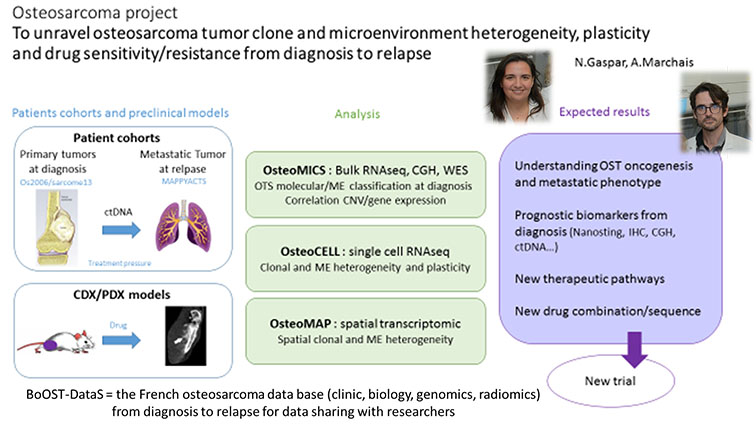
The second axis on osteosarcoma research led by N Gaspar and A Marchais builds on patient samples at diagnosis and relapse as well as PDX models, investigates osteosarcoma transcriptomic, genomic and immune landscape, at single cell and spatial level via high throughput sequencing, bioinformatic integration and experimental validations. The ultimate goal being to identify druggable targets, and validate them in vitro and in vivo (PDX models) before translation into clinical trial.
This axis operates the implementation of the national osteosarcoma database BoOST-DataS with clinic, biologic, genomic and radiomics osteosarcoma patient data to be shared with the scientific community.
These objectives are accompanied by parallel efforts to establish a repository of patient-derived xenograft models of advanced pediatric tumors, most are included in the European ITCC-P4 consortium, which provide unique opportunities for the preclinical evaluation of candidate therapeutic approaches.
Collaborations: University of Oklahoma, Stanford School of Medicine, Harvard Medical School, USA; University of Kuala Lumpur, Malaysia; Institut Pasteur, Paris
