Cell division and genomic stability
This group belongs to:
UMR 9019 Genome Integrity and Cancers - Genome integrity, immune response and cancer
Cancer is a group of diseases originating from uncontrolled cell proliferation. A common hallmark of cancer cells is the presence of genetic alterations (point mutations, chromosome gain or loss, etc.) whose progressive accumulation is correlated with disease aggressiveness. Cell proliferation is a physiological process that allows tissue renewal and homeostasis during adulthood. This process is normally finely tuned by basic molecular machinery driving first the complete duplication (copy) of the genome then its equal segregation into two nascent daughter cells. Additionally, surveillance mechanisms (checkpoints) verify the integrity of the genome at each step, ensuring that daughter cells will receive identical genetic content to the parental cell. Our team aims to determine how genetic alterations (DNA damage, under-replicated regions..) can eventually persist up to the time cells enter into mitosis and divide, challenging the integrity of the genetic content transmitted to the progeny and affecting cell properties and fate. We focus our current investigations on three axes.
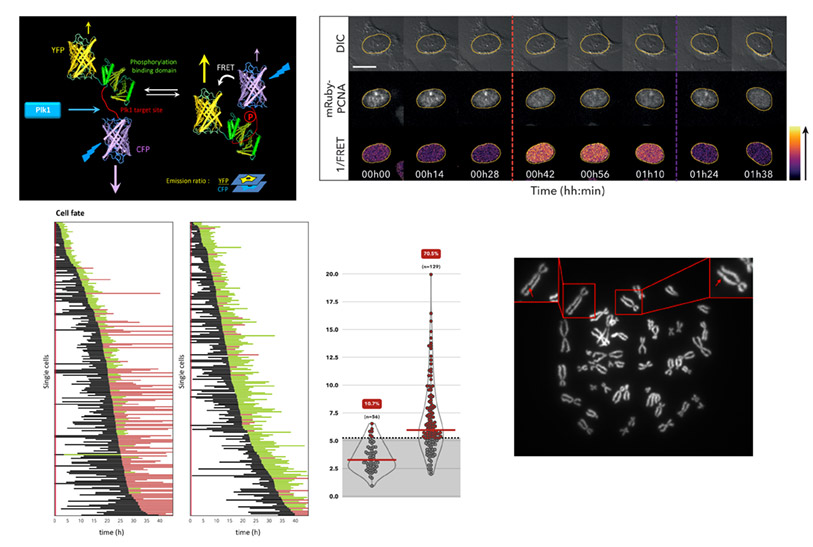
- First, we aim to decipher the signaling pathways controlling entry into mitosis and ensuring that it is normally coordinated with the completion of genome duplication. Our recent work has demonstrated that Polo-like kinase 1 (Plk1) is required for commitment into mitosis in human cells and that its temporal activation is controlled by the DNA replication machinery.
- Second, we investigate in which contexts the deregulation of pro-mitotic factors, as described in various human cancers, can trigger a premature cell division and contribute to incorrect genome transmission.
- Third, we aim to determine by which molecular mechanism(s) genetic alterations can override efficient DNA surveillance mechanisms, notably in non-cancerous cells, challenging the maintenance of genetic integrity.
Altogether, we expect to provide important knowledge on the mechanisms at work early on during tumorigenesis in the establishment/amplification of genetic alterations.
To achieve our objectives, we are developing powerful genetic tools such as FRET (Förster Resonance Energy Transfer)-based sensors of kinase activities, combined with advanced fluorescence-based live cell imaging assays, as well as bioinformatics and statistical analyses of the behavior of individual cells in a population in order to decipher the mechanisms mentioned above in a non-invasive way.
Currently, there are opportunities to join our group as a Master 2 or PhD student.