Metabolic plasticity in health and disease
This group belongs to the UMR 9018 - Metabolic and systemic aspects of oncogenesis for novel therapeutic approaches (Metsy)
Our team is studying the role of mitochondria in the metabolic plasticity of cancer cells with the aim of discovering new biomarkers and proposing new therapeutic strategies that could improve the tumour response to cancer treatments. Indeed, mitochondria, which are energy production factories in the cell, play an important role in tumorigenesis and the response of tumour cells to anticancer treatments.
Research topics
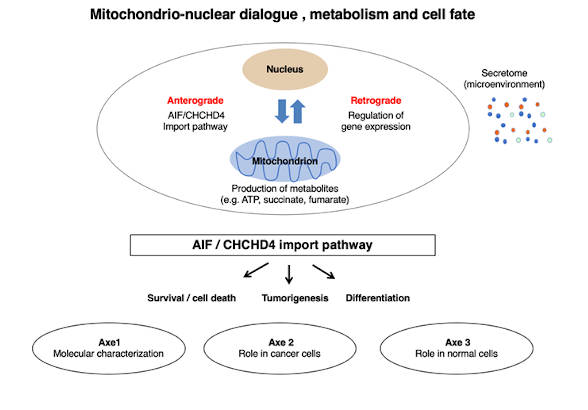
Metabolic plasticity is a fundamental ability that allows cells to adapt their metabolic status to specific needs during proliferation, differentiation, activation in response to various stimuli or in response to stress conditions. In this context, mitochondria, which are major players through their contribution to metabolism, communicate with other cellular compartments, particularly with the nucleus. Thus, mitochondrial activity is finely regulated through a bidirectional dialogue with the nucleus. In the first place, an anterograde dialogue (from the nucleus to mitochondria) controls the mitochondrial biogenesis by allowing the import of 1000 to 1500 proteins into one of the four sub-compartments of the organelle, namely the inner and outer membranes, the mitochondrial matrix and the intermembrane space. In return, through the production of ATP and other metabolites, mitochondria establish a retrograde dialogue with the nucleus (from mitochondria to the nucleus), which impacts on the expression of genes necessary for, among other things, cellular metabolic programs and secretion activities involved in systemic pathways.
We are developing an integrative research program for a better understanding of the mitochondrio-nuclear dialogue mentioned above. Our work will focus on the mitochondrial import pathway AIF/CHCHD4, which regulates the import of a family of proteins involved in cell survival, adaptation to stress and response to apoptotic stimuli. Our objectives will be 1/ to better understand the role of these proteins in the metabolic plasticity of normal and tumour cells and 2/ to exploit the acquired knowledge for the generation of new molecular tools, the discovery of new biomarkers and the proposal of new therapeutic targets and anti-cancer molecules to be combined with conventional therapeutic strategies (radio- or chemotherapy).
This research program is developed within 3 axes:
Axis n°1: Molecular characterization of the AIF/CHCHD4 complex. In order to characterize the AIF/CHCHD4 complex, we are aiming to understand signaling pathways that regulate this complex, to generate tools to target mitochondria and study the interaction between AIF/CHCHD4, and to identify, through high-throughput screening, molecules that could modulate the AIF/CHCHD4 import pathway and thus affect metabolism, cancer cell fate and stress signaling response.
Axis n°2: Impact of the AIF/CHCHD4 pathway on tumour metabolism. Since mitochondria are now recognized as key players in the metabolic plasticity of various cancer cells and in the sensing and propagation of signals generated either by tumor cells or by the tumor microenvironment, within this axis, through genetic and pharmacological manipulations, we are studying the role of the AIF/CHCHD4 pathway in tumor metabolism, tumorigenesis, response to therapeutic agents and resistance to anticancer treatments.
Axis n°3: Metabolic bases of stem cell differentiation. Metabolic plasticity is fundamental for the control of stem cell homeostasis and the regulation of their ability to differentiate into specific cell lineages. In this axis, we 1/ study the regulation of the AIF/CHCHD4 import pathway during stem cell differentiation and 2/ evaluate its impact on the ability of mitochondria to adapt their activity to the metabolic and bioenergetic needs of stem cells that undergo differentiation.
Historically, the team hosts one of the axes of the former UMR 8203 (2011-2019) led by Lluis M. Mir. More precisely, following the work carried out over three decades, an activity centered on the study of the interactions of electric and electromagnetic fields is being pursued. The aim is to modify the permeability of cell membranes and organelles, to exploit all the resulting applications (electrochemotherapy, non-viral gene therapy, irreversible electroporation in oncology, etc.) and to study the bases and fundamental mechanisms.
Our laboratory is equipped with a Seahorse analyzer (Seahorse, Agilent), which allows simultaneous and real-time measurement of the cell's main energy pathways. Our work is backed up by the platforms managed by Gustave Roussy's UMS AMMICA (UMS 3655) as well as the CIBLOT high throughput screening platform, labeled IBISA, which is part of Criblage@ParisSaclay and Chembiofrance's national infrastructure.