14th November 2023
A neural organoid with an immune environment
French, Singaporean and British researchers, led by Prof. Florent Ginhoux, head of a research team at Gustave Roussy/Inserm, have succeeded in demonstrating in a neuronal organoid the role of the brain's immune environment in its formation and development. The development of these three-dimensional structures integrating neuronal cells and the immune environment is, to date, one of the most complete in vitro models of the human brain. This work is published in Nature.
At Gustave Roussy, these organoids are used to model the development of childhood brain cancers, to understand their mechanisms and discover new avenues of treatment.
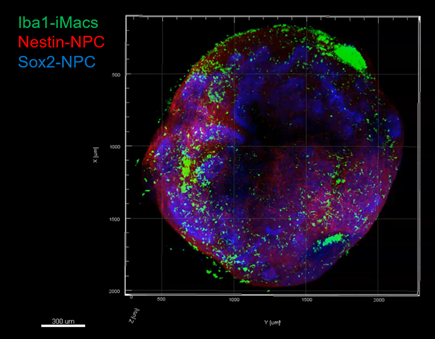
Neural organoid with immune environment magnified twice on the left, 20 times on the right: green macrophages, red and blue neural progenitor cells (fluorescence microscopy).
"Although microglial cells, immune cells derived from the evolution (differentiation) of primitive macrophages present in the embryonic brain, are known to contribute to multiple aspects of brain development and function, their precise role remains poorly understood and little studied", says Prof. Florent Ginhoux, director of a research team at Gustave Roussy/Inserm and Senior Principal Investigator at A*STAR’s Singapore Immunology Network (SIgN) . The use of neuronal organoids to study their functions is one of the avenues currently favored by research.
With their 3D structure, the function and properties of these organoids are close to those of a real organ, but not quite as advanced. They measure just one millimeter and have no thoughts, consciousness or emotions. By generating neural organoids from human induced pluripotent stem cells (iPS cells), it is possible to model some of the key features of early human brain development. "However, current approaches do not include microglial cells," explains Prof. Florent Ginhoux.
The international team of researchers led by Prof. Florent Ginhoux has succeeded in producing a new type of model: neuronal organoids with microglia, by cultivating together organoids and primitive-type macrophages, all generated from the same culture of iPS induced stem cells.
Organoids and primitive macrophages are prepared separately. It takes around 25 days to obtain them. The macrophages are then placed in contact with the organoids for a further 15 to 20 days.
In the model developed by the researchers, macrophages colonized the organoids. In this 3D environment, in contact with immature neuronal cells, they differentiated into microglial cells expressing the genes and functions specific to this cell type. These microglial cells proved capable of controlling the differentiation of neuronal precursors (so-called neuronal progenitor cells), thus limiting their multiplication (proliferation), while promoting synapse creation (synaptogenesis) and axon growth (axonogenesis), two key elements in the transmission of the nerve message from neuron to neuron.
A discovery within a discovery
Prof. Florent Ginhoux's team also observed that the organoids' microglial cells contain high levels of perilipin-2, a molecule belonging to a family of proteins that coat lipids - including cholesterol - in droplets, enabling them to be stored in and exported from the cells. Armed with these perilipin-2-laden droplets, microglial cells facilitate cholesterol transport to the organoids. The neural progenitor cells that absorb this cholesterol undergo metabolic reprogramming as they differentiate into nerve cells.
The approach developed by Prof. Florent Ginhoux and his colleagues has significantly advanced the complexification of organoid models by integrating microglial cells. This progress is illustrated by the discovery of a key lipid-mediated pathway between microglia and neural progenitor cells, essential for the synthesis of new neurons.
"With microglia cells incorporated, the neural organoids we have succeeded in generating are a new, more complete 3D model, closer to reality. We know that the immune system plays a fundamental role in the development of cancers, so at Gustave Roussy we're going to use them to better understand and discover the mechanisms that regulate the development of pediatric brain tumors", concludes Prof. Florent Ginhoux.
This work has been supported by A*STAR, SIgN and the Gustave Roussy Foundation's "Curing childhood cancer in the 21st century" campaign.
![]() ► Read the press release in PDF
► Read the press release in PDF
Nature
iPSC-microglia promote brain organoid maturation via cholesterol transfer
Advanced publication on ligne the 1st November 2023
